What Is the Solvent Concentration Inside the Cell
A solution in which there is a higher concentration of water molecules solvent inside a cell than outside a cell. What is the solvent concentration inside the cell.

What Is Osmosis Plasma Membrane Membrane Biology
Which would prevent a plant from growing.

. The Gizmo only displays the solute concentrations. What is the solvent concentration outside the cell. To calculate percentage concentration divide the number of solute particles by the total number of particles solute solvent and then multiply by 100.
To calculate the solvent concentration divide the number of solvent particles by the total number of particles and then multiply by 100. 3 Show answers Another question on Biology. When she was six months old she used to grab a block with her.
A hypertonic solution is one that has a higher solute concentration outside the cell than inside. Natalie had random hand movements when she was two months old. In what situation does the cell get smaller.
A solution with a low solute concentration has a high water concentration. Calculate the Molarity of the solution. Provide an example of passive transport including a description of how it works.
3 Show answers Another question on Biology. Knowing the amount of solute inside and outside the cell as you have written in your cell now figure out the amount of water solvent inside the cell and outside. Psychology questions and answers.
The Gizmo only displays the solute concentrations A. What is osmosis ans. The concentration of a solute is calculated using Molarity.
A hypotonic solution is the one that has a higher solute concentration inside the. The Gizmo only displays the solute concentrations Note. To calculate the solvent concentration divide the number of solvent particles by the total number of particles and then multiply by 100.
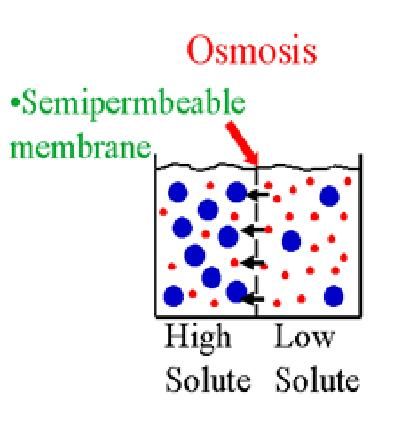
The more solute the less solvent ie. This movement is caused by a concentration gradient created when there are different solute concentrations inside and outside the cell. Osmosis is a passive transport system meaning it requires no energy.
Rhizobia use the legumes for food. When the concentration of a solvent is the same inside the cell as it is outside the cell it is called Provide an example of active transport including a description of how it works in your own words. Check that the Solute outside is 10 and the Initial cell volume is 40.
It doesnt matter what dissolved. A concentration gradient exists when there is a region of high. The outside of the cell has more salt concentration than inside the cell.
Inside the cell there is solute and solvent must add up to 100. Up to 24 cash back Calculate. Solute and _ solvent.
Concentration solute total particles 100. The Gizmo only displays the solute concentrations. The concentrationof a solute is the amount of solute particles in a given amount of solvent.
Pure water has the highest water concentration. When there is more solutes outside the cell to begin with so the solvent inside the cell goes outside and the cell shrinks During osmosis solvent particles move from an area of ______ concentration to an area of ___________ concentration. There is more solute in the solution surrounding a cell than inside resulting in a greater amount of water solvent inside the cell.
The concentration of mixtures is important because. If the concentration of SOLUTE particles is higher INSIDE the cell than outside the cell there will be a net flow of SOLVENT particles flowing _____ the cell which will gardually lower the solute concentration and cause the cell to _____. First find the number of moles of solute by multiplying the amount of NaCl by the molar mass.
The concentration of ions and other solute molecules is higher inside the cell than outside it so water moves into the cell via osmosis. Osmosis is diffusion of water or solvent through a semi-permeable membrane from the region of lower solute concentration to that of higher solute concentration ie down the. The solute is the NaCl.
However solvent particles usually water molecules CAN move into and out of the cell. What would most likely happen to the. Solute concentration is a term used to describe mixtures and defines how much of one substance called the solute is dissolved in another referred to as the solvent.
An isotonic solution is one that has the same concentration of solutes both inside and outside the cell. What is the solvent concentration outside the cell. It depends on the conditions.
In a hypertonic solution a higher concentration of water is inside of the cell. It causes water to move in and out of cells depending on the solute concentration of the surrounding environment. To calculate the solvent concentration divide the number of solvent particles by the total number of particles and then multiply by 100.
A 50 L aqueous solution contains 20. Legumes a type of plant require rhizobia a type of soil bacteria to survive since these organisms fix nitrogen during photosynthesis. There are a number of ways to describe concentration depending on need and can involve weight volume or molecular mass.
So here we have initial volume less than 50 this implies that our solute concentration inside the cell is larger than solute concentration outside and hence solvent concentration inside the cell is lesser than solvent concentration outsidesince solute solventsolution so if one increases other have to decrease. Molarity M moles of soluteLiters solution. What causes a solution to be hypertonic.
What is the solvent concentration inside the cell.
How Does Osmosis Relate To Solute Concentration Socratic

How To Understand Diffusion Osmosis Science Cells Biology Resources Teaching Cells

Types Of Solutions Infographic Diagram Including Isotonic Hypertonic Hypotonic And Relation Betwe Middle School Science Activities Teaching Cells Science Cells
0 Response to "What Is the Solvent Concentration Inside the Cell"
Post a Comment